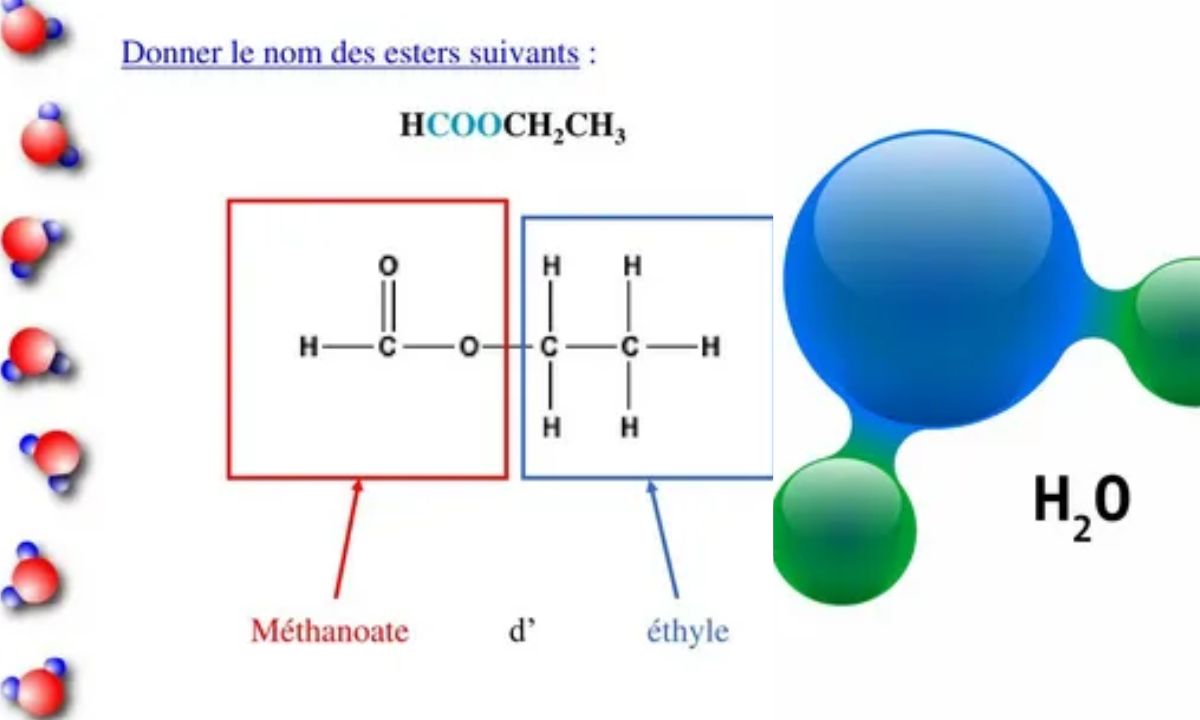
HCOOCH CH₂ H₂O is an intriguing chemical substance with significant importance in contemporary chemistry. This unique molecule combines a formate group, methylene bridge, and water molecule, creating a versatile structure with numerous applications. Scientists and researchers across pharmaceutical, polymer, and environmental industries rely on its distinctive properties.
The compound’s growing importance in sustainable chemistry and drug development makes it essential for students and professionals to understand its behavior. This comprehensive guide explores everything from its basic structure to cutting-edge applications.
Recent studies show increasing interest in HCOOCH CH2 H2O as industries seek greener chemical alternatives. Its biodegradable nature makes it particularly valuable in eco-friendly manufacturing processes.
Structure and Composition of HCOOCH CH₂ H₂O
HCOOCH CH2 H2O features three key components that determine its chemical behavior:
- Formate group (HCOO) – Derived from formic acid, providing reactivity
- Methylene bridge (CH2) – Single carbon with two hydrogens acting as a linker
- Water molecule (H2O) – The H₂O molecule can act either as a solvent or emerge as a byproduct in chemical reactions.
These elements combine to create a molecule with both polar and non-polar regions. The oxygen atoms contribute to hydrogen bonding capabilities, while the carbon chain provides limited hydrophobic properties.
Advanced spectroscopic techniques like NMR spectroscopy and infrared analysis help scientists confirm its structure in various mixtures and solutions. These methods reveal important details about bond angles and electron distribution.
Physical and Chemical Characteristics of HCOOCH CH₂ H₂O
Physical Properties
The distinct physical traits of HCOOCH CH₂ H₂O enhance its utility across numerous applications:
- Appearance: Colorless liquid at room temperature
- Odor: Mild, somewhat sweet aroma
- Density: Approximately 1.15 g/cm³
- Solubility: It dissolves well in water and other polar organic liquids.
Temperature significantly affects its viscosity, with lower temperatures causing increased thickness. This property proves useful in temperature-sensitive applications where flow control matters.
Chemical Properties
The chemical reactivity of HCOOCH CH2 H2O stems from its functional groups:
- Hydrolysis Behavior: It decomposes when exposed to acidic or basic aqueous environments.
- Nucleophilic substitution – The formate group participates in various replacement reactions
- Hydrogen bonding – Forms networks with water and alcohols
- Thermal Stability: It undergoes decomposition at temperatures exceeding 180°C.
Its pH stability range of 4-8 makes it compatible with many biological systems. Outside this range, degradation occurs rapidly, yielding simpler compounds like formic acid and methanol.
Synthesis and Production Methods
Esterification Reaction
The most common synthesis pathway involves direct esterification of formic acid with appropriate alcohols:
HCOOH + CH3OH → HCOOCH3 + H2O
This reaction typically necessitates an acid catalyst such as sulfuric acid and requires moderate heating between 50°C and 70°C. Yield optimization involves careful water removal to drive equilibrium toward product formation.
Hydrolysis of Larger Esters
Larger formate esters undergo controlled hydrolysis to produce HCOOCH CH2 H2O:
- Select suitable parent compound (typically a diester)
- Add precise amount of water with buffer solution
- Monitor reaction using chromatography techniques
- Isolate product through distillation or extraction
This method achieves higher purity but typically produces smaller quantities compared to direct synthesis.
Industrial Catalytic Processes
Modern industrial production employs heterogeneous catalysts like zeolites and metal oxides. These processes offer several advantages:
| Process Type | Catalyst | Yield | Energy Usage | Environmental Impact |
| Traditional | H2SO4 | 65% | High | Moderate |
| Zeolite | ZSM-5 | 78% | Medium | Low |
| Metal Oxide | Al2O3-TiO2 | 82% | Low | Very Low |
Large-scale production typically employs continuous flow reactors rather than batch processes to maximize efficiency.
Applications of HCOOCH CH2 H2O
Pharmaceutical Industry
In the pharmaceutical sector, HCOOCH CH₂ H₂O fulfills several key roles.
- Drug carrier for controlled-release medications
- Stabilizing agent in certain antibiotic formulations
- Reaction medium for sensitive synthesis steps
- Building block for complex pharmaceutical intermediates
Its low toxicity profile makes it particularly valuable for medications requiring strict safety standards.
Polymer Chemistry
The polymer manufacturing industry also employs this compound effectively.
- Cross-linking agent for enhanced polymer strength
- Chain terminator in controlled polymerization reactions
- Modifier for improving flexibility in rigid plastics
- Biodegradable component in environmentally friendly materials
These uses have significantly contributed to the advancement of eco-friendly packaging solutions, helping to decrease reliance on petroleum-based materials.
Organic Synthesis
Synthetic chemists rely on HCOOCH CH2 H2O for:
- Functional group transformation
- Carbon chain extension
- Protecting group chemistry
- Stereoselective reactions
Its versatility enables the creation of complex molecules with precisely controlled structures.
Environmental Chemistry
Environmental applications include:
- Green solvent alternative to traditional options
- Biodegradable plasticizer for eco-friendly materials
- Reagent in water treatment processes
- Component in sustainable agricultural products
As industries focus increasingly on sustainability, HCOOCH CH2 H2O’s biodegradability makes it an attractive choice.
Safety and Handling Precautions HCOOCH CH2 H2O
Working safely with HCOOCH CH2 H2 O requires proper equipment and knowledge:
- Always ensure the use of suitable personal protective equipment (PPE), such as gloves and safety goggles
- Ensure adequate ventilation in work areas
- Keep the substance stored in well-sealed containers, positioned away from heat or ignition sources
- Follow all disposal regulations for chemical waste
Standard safety data sheets classify it as moderately hazardous, with primary concerns being skin and eye irritation upon direct contact.
Comparison with Similar Compounds HCOOCH CH2 H2O
Understanding how HCOOCH CH2 H2O compares to related compounds helps contextualize its properties:
| Compound | Structure | Key Difference | Primary Use |
| Methyl Formate | HCOOCH3 | Simpler structure | Flavoring |
| Formic Acid | HCOOH | More acidic | Industrial cleaner |
| Glycol Formate | HCOOCH2CH2OH | Additional hydroxyl | Pharma intermediate |
| HCOOCH CH2 H2O | HCOOCH CH2·H2O | Water component | Versatile reagent |
The water component in HCOOCH CH2 H2O contributes significantly to its unique reactivity profile, distinguishing it from similar esters.
Future Research and Developments HCOOCH CH2 H2O
Current research focuses on expanding HCOOCH CH2 H2O applications in:
- Nanomaterial synthesis for advanced electronics
- Biofuel production as a processing agent
- Medicinal chemistry for targeted drug delivery
- Biodegradable plastics for reducing environmental impact
These innovations promise to further increase the compound’s importance in coming years.
Frequently Asked Questions
What is the primary industrial application of HCOOCH CH₂ H₂O?
Primary industrial applications include polymer modification and pharmaceutical manufacturing.
Is HCOOCH CH2 H2O environmentally friendly?
Yes, it biodegrades relatively quickly and has a lower environmental impact than many alternatives.
How stable is HCOOCH CH2 H2O at room temperature?
It remains stable for months when properly stored in sealed containers away from extreme pH conditions.
Can HCOOCH CH2 H2O cause allergic reactions?
Allergic reactions are rare but possible with repeated exposure in sensitive individuals.
What personal protective equipment is needed when handling this compound?
Nitrile gloves, safety goggles, and lab coat are recommended for safe handling.
How can HCOOCH CH₂ H₂O be detected within a chemical mixture?
IR spectroscopy and NMR analysis are the most reliable identification methods.
Is HCOOCH CH₂ H₂O suitable for use in food-related products?
Limited food applications exist due to regulatory status; primarily used in non-consumable products.
Conclusion
HCOOCH CH2 H2O represents an important compound at the intersection of traditional and green chemistry. Its unique structure combines the reactivity of formate esters with the versatility introduced by its methylene bridge and associated water molecule. This combination creates exceptional utility across multiple industries.
As sustainability concerns grow, HCOOCH CH2 H2O’s biodegradable nature and relatively low environmental impact position it as an increasingly valuable chemical resource. Ongoing research continues to discover new applications and improved synthesis methods, ensuring its place in the future of chemical science.